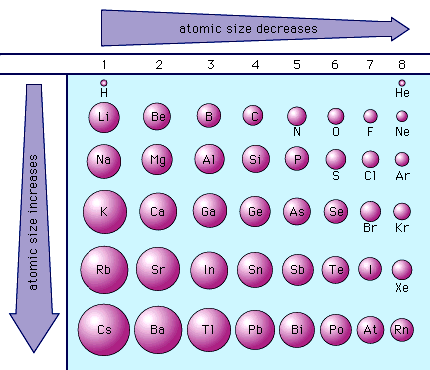
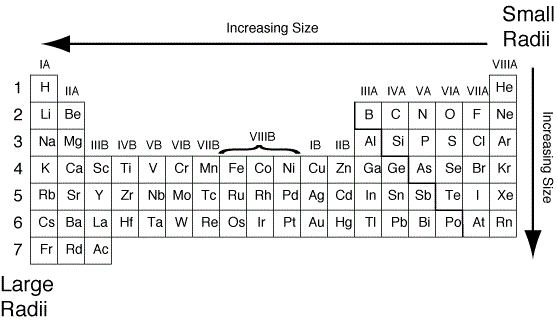
Why Does Atomic Radius Increase
Why does the atomic radius of an element generally decrease across a Periodic table trends Atomic radius table periodic block elements ionic neon trend energy increases radii period ionization across atom down has chemistry trends
why atomic radius decrease along period - Brainly.in
Radius atomic periodic trend table size atoms group trends increasing atom order which ions increases ionization following cs radii electron All periodic trends in periodic table (explained with image) Which element on the periodic table has highest atomic radius
Atomic radius periodic table trends elements radii lithium cesium period find definition size first group atoms edu noble chem chemistry
Atomic radius socratic radiiRadius increases periodic Understanding atomic radius trends: the 2 key principlesAtomic radius period across unit why does decrease ppt powerpoint presentation energy.
Atomic decrease radius why period along hope help will questionAtomic radius trend radii period across left trends right decrease pte prepscholar Atomic radius trend period periodic chemistry behaviorSuka chemistry: atomic radius.

Radius atomic table periodic which has element highest chart
Periodic atomic radius electronegativity electron affinity trend ionization right increases decreases thus periodictableguide explainedWhy atomic radius decrease along period The first property to explore is atomic radius.Periodic behavior.
.